FDA LISTING INC: EXPERIENCED ADVISORS FOR FULL-SERVICE FDA REGISTRATION & COMPLIANCE
+1 212 444 8202
FDA Food and Dietary Supplement Labeling Requirements
Click the button below to see the service steps in an easy-to-follow graphic format.
The FDA has set clear guidelines for labeling food and dietary supplements to protect public health. Brand owners, manufacturers, and importers of food items must follow these mandatory FDA regulations, ensure their labels provide accurate details, and remain updated on new changes. This is important because food labeling inaccuracies and possible FDA enforcement actions can significantly damage a food and dietary supplements brand’s market position.
Two primary food and supplement label considerations are 1) Food product ingredients and 2) Food product labels.
What are the Food Ingredient Considerations?
FDA has three pillars of regulations for food ingredients, which are listed below.
1) Generally Recognized as Safe (GRAS): a food substance is exempt from FDA premarket review because it is generally recognized as safe by qualified experts under the intended conditions of use.
2) Food additives: any substance added to food to improve the taste, texture, appearance, or nutritional value must be pre-approved unless generally considered safe.
3) Color additives: the FDA must pre-approve any color additive, regardless of whether it is derived from natural sources
The GRAS Substances (SCOGS) Database contains thousands of food substances, including additives, preservatives, colorings, flavor enhancers, and emulsifiers. As a general rule, food manufacturers must review and verify their substances in the GRAS database (not included in the label review service). Verifying the ingredients primarily indicates that the FDA has deemed the substance safe for use in food products, which can reassure consumers about its safety.
What are FDA Label Considerations?
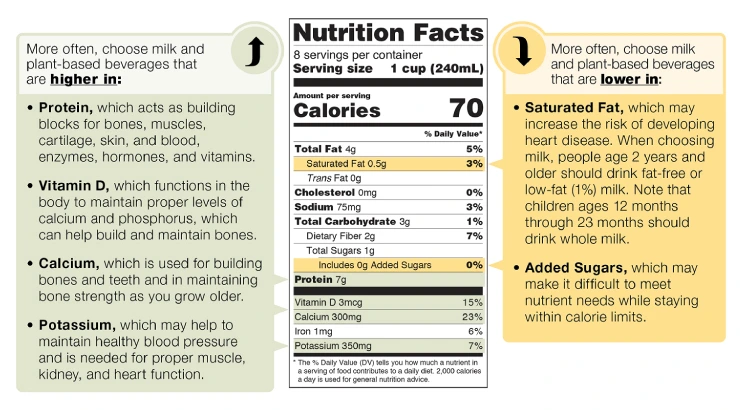
According to the new FDA regulation, food, beverage, and dietary supplement labels must contain Nutrition or Supplement Facts information that complies with new FDA requirements concerning the label formatting, nutrient names and the exact amounts, and percent daily value calculation. Food or supplement labels generally consist of four different sections described below:
● Principal Display Panel: a brand name, statement of Identity, and net quantity.
●Information Panel: ingredients, directions for use, warnings, food allergen declarations, manufacturer’s name and address, and country of origin.
●Nutrient or Supplement Panel: nutrient components labeling and serving sizes.
● Labeling Claims: nutrient content, health, or other regulated claims
Nutrition or Supplement Facts Panel: Calculate and determine the serving size and the daily values in the U.S. metric system and prepare the nutrition or supplement facts panel. FDA has approved multiple chart formats (tabular, simplified, linear, etc.) to accommodate various label sizes.
Ingredient Statement: Review and advise for changes to the order or the nomenclature of the statement to follow an FDA-compliant and consumer-friendly ingredient statement.
Labeling Claims: Review and guidance for nutrient content claims, organic claims, health claims, and other regulated (i.e., pasteurized, natural, fresh, etc) claims that appear on the product label.
Label Layout and Format: Redesign and suggest a finished label that fits into the product profile in size, shape, layout, and formatting.
Other Considerations: include Allergen statements, structure and function claims, and nutrient claims.
Labeling issues are often the primary cause of delays in FDA shipment clearance. If you seek experienced regulatory specialists to evaluate your food labels before entering the US market, you are in the right place. FDA Listing Inc. conducts label reviews for food products and dietary supplements according to FDA labeling guidelines and regulations. This ensures that your product labels meet mandatory FDA requirements and are FDA-compliant. We offer a free 30-minute consultation to discuss our findings and provide guidance on necessary changes.
Service Details & Fees
Food Label Review
Time Required
Current Label Assessment
60 Days Unlimited Revisions
Discount on Multiple Labels
495 USD
5-7 Days
_______ Related Services _______
Frequently Asked Questions (FAQ)
No, the FDA does not accept or review food product labels. Instead, it is the manufacturer’s responsibility to ensure the product labels are compliant.
Before entering the US market, you must register the manufacturing facility only for common food products. You may also submit product identifiers (SIDs) for specific food items, such as shelf-stable, low-acid, or acidified canned food.
No, the FDA does not accept or review your dietary supplement labels. Instead, it is the manufacturer’s responsibility to ensure the product labels are FDA-compliant.
No, the FDA (U.S. Food and Drug Administration) label does not automatically meet the requirements for importing food into Canada, and vice versa.
The FDA does not legally mandate “may contain” or “produced in a facility that also processes” statements for possible cross-contamination. However, manufacturers often voluntarily include these warnings to protect consumers with allergies and to minimize liability.
The FDA has not specified a required number of samples for analysis. The manufacturer is responsible for assessing the variability of their product(s) and determining the appropriate number of samples to ensure accurate nutrient information.
Frequently Asked Questions (FAQ)
No, the FDA does not accept or review food product labels. Instead, it is the manufacturer's responsibility to ensure the product labels are compliant.
Before entering the US market, you must register the manufacturing facility only for common food products. You may also submit product identifiers (SIDs) for specific food items, such as shelf-stable, low-acid, or acidified canned food.
No, the FDA does not accept or review your dietary supplement labels. Instead, it is the manufacturer's responsibility to ensure the product labels are FDA-compliant.
No, the FDA (U.S. Food and Drug Administration) label does not automatically meet the requirements for importing food into Canada, and vice versa.
The FDA does not legally mandate "may contain" or "produced in a facility that also processes" statements for possible cross-contamination. However, manufacturers often voluntarily include these warnings to protect consumers with allergies and to minimize liability.
The FDA has not specified a required number of samples for analysis. The manufacturer is responsible for assessing the variability of their product(s) and determining the appropriate number of samples to ensure accurate nutrient information.
Service Details & Fees
Food Label Review
545 USD
Time Required
5-7 Days
Current Label Assessment
60 Days Unlimited Revisions
Discount on Multiple Labels
_______ Related Services _______




