FDA LISTING INC: EXPERIENCED ADVISORS FOR FULL-SERVICE FDA REGISTRATION & COMPLIANCE
+1 212 444 8202
FDA Requirements for Hand Sanitizers and Other Antiseptic OTC Drugs
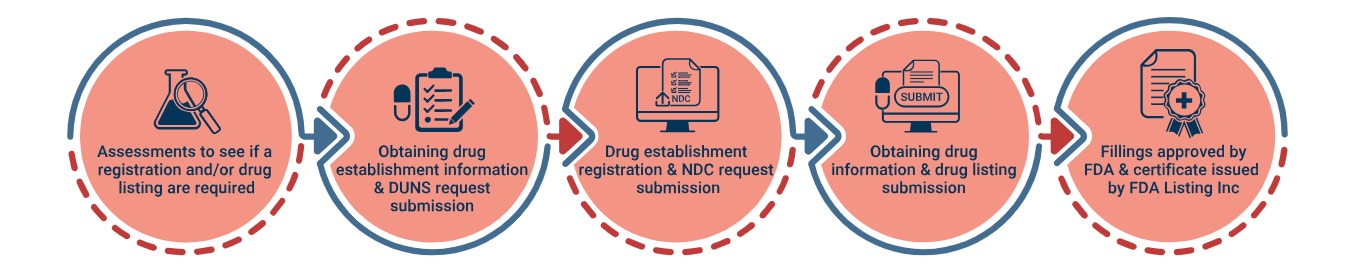
Hand Sanitizers and most form of antiseptic/antibacterial products are considered over-the-counter (OTC) drugs by the FDA. In order to introduce an OTC drug to the U.S. market, the manufacturers must fulfill certain FDA requirements that are summarized below.
OTC Drug Manufacturers:
- Follow the relevant OTC monograph published by the FDA in compounding/formulation of the hand sanitizer (as well as GMP).
- Register the Drug Manufacturing Establishment with the FDA and assign a US agent (official correspondent)
- Obtain the National Drug Code , NDC
- List the Drug with the FDA and submit the final label of the drug product
- Comply with the FDA Drug Labeling Requirements
U.S. OTC Drug Importer / Private Label Distributor:
- Obtain the National Drug Code , NDC
- List the Drug with the FDA and submit the final label of the drug product
- Comply with the FDA Drug Labeling Requirements
The registration and listing process can take 6-8 workdays depending on how quickly you can provide the FDA-required information. Note, FDA may not need to conduct site inspection prior to allowing you to sell in the US market, however the FDA can inspect manufacturing plant at any point to verify the GMP (Good Manufacturing Practice) compliance.
FDA's Temporary Policies for Alcohol-Based Hand Sanitizers in response to COVID-19
In the light of the current dynamic due to the COVID-19 pandemic the FDA has recently published two guidance documents for industry for compounding pharmacies as well as companies not typically regulated by the FDA .

What are key points in FDA new policy?
The hand sanitizer should be made according to specific formulations and by using U.S. Pharmacopoeia-grade ingredients listed below:
o Isopropyl Alcohol (75%, volume/volume) or Alcohol (ethanol 80% volume/volume) in a water containing solution
o Glycerol (1.45% volume/volume)
o Hydrogen peroxide (0.125% volume/volume)
o Sterile distilled water / boiled and cooled water
Manufacturer should utilize the most accurate method for analyzing the alcohol content of each batch of finished product. The sanitizer products must be prepared under sanitary conditions that have appropriate equipment’s in place.
OTC Drug products that do meet such FDA conditions must follow the FDA’s standard drug requirements for approval (OTC monograph, new drug application-NDA, etc.).
FDA Listing Inc. is helping companies with the U.S. FDA compliance. If you are planning to market hand sanitizers or any form of antiseptic products, we can help you in fulfilling FDA requirements. Feel free to call us at +1 929-376-7870 or chat with our regulatory advisors for immediate assistance.
_______ Related Services _______




