FDA LISTING INC: EXPERIENCED ADVISORS FOR FULL-SERVICE FDA REGISTRATION & COMPLIANCE
+1 212 444 8202
FDA GUDID and UDI Compliance for Medical Devices
Click the button below to see the service steps in an easy-to-follow graphic format.

The FDA has started the Unique Device Identification (UDI) system to identify and track all medical devices marketed in the United States. The FDA’s UDI rule requires device labelers, typically manufacturers or brand owners, to include a UDI on device labels and packages.
As of September 24, 2022, labelers of all device classes I, II, and III sold in the U.S. must submit their device information on the FDA Global Unique Device Identification Database (GUDID) and obtain the UDI. The FDA system also considers limited UDI exceptions and alternatives.
Key Components of UDI
● To generate UDIs, device labelers must submit the device information to GUDID.
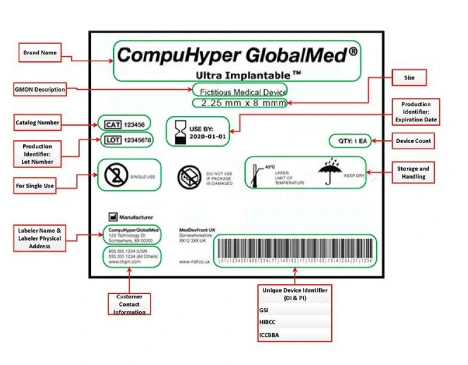
● UDI is a unique code consisting of a Device Identifier (DI) and a Production Identifier (PI) portion created in human—and machine-readable format.
● The DI is a mandatory part of a UDI issued by an accredited FDA agency and includes a fixed portion that identifies the labeler and device version or model.
●The PI is provisional and includes a variable portion that identifies device specifications or production details, such as manufacturing and expiration date, serial number, batch, or lot number, etc., which can facilitate the search in the GUDID database.
UDI Submission Steps
1) Obtain the DUNS number & validate labeler information
2) Assign regulatory contact & request a GUDID account
3) Verify GMDN standard codes & create UDIs
4) Submit UDIs & device information on GUDID
How a UDI recognized on a medical device label?
1) The device label: some labels may include ‘UDI’ near their code.
2)Two formats: the UDI is usually presented as a human-readable number and in a machine-readable barcode format.
The following example of a UDI illustrates a sample device label.
GUDID Database
The FDA administers the Global Unique Device Identification Database, or Access-GUDID, an extensive catalog for all devices featuring a unique device identifier (UDI). GUDID exclusively contains the device identifier (DI) portion of the UDI, serving as the primary means to access device information in the database.
Although GUDID doesn’t encompass production identifiers (PIs), it does include PI flags to indicate which PI attributes are present in the UDI. Moreover, the AccessGUDID Database, a universal UDI database, consists of the Global Medical Device Nomenclature (GMDN) Code, specifying its status as Active or Obsolete.
FDA Listing Inc. is committed to assisting companies in meeting the FDA’s UDI requirements and facilitating timely and successful data submission to GUDID. Feel free to call or email us if you need assistance.
Service Details & Fees
GUDID Account
UDI Submission
Time Required
30-Min Expert Consultation
Discount on Multiple UDIs
395 USD
195 USD
4-6 Days
_______ Related Services _______
Frequently Asked Questions (FAQ)
U.S. FDA requires filling of Prior Notice before food shipments enter the United States. This will include Prior Notice for food samples for trade shows or consumption. After filling the Prior Notice, you will be assigned with a confirmation number that will be used by your U.S. customs broker for the shipment release. A Prior Notice can also be filed by your own compnay or international transit firms as well as shipment companies (DHL, FedEx, TNT, etc).
U.S. FDA requires filling of Prior Notice before food shipments enter the United States. This will include Prior Notice for food samples for trade shows or consumption. After filling the Prior Notice, you will be assigned with a confirmation number that will be used by your U.S. customs broker for the shipment release. A Prior Notice can also be filed by your own compnay or international transit firms as well as shipment companies (DHL, FedEx, TNT, etc).
U.S. FDA requires filling of Prior Notice before food shipments enter the United States. This will include Prior Notice for food samples for trade shows or consumption. After filling the Prior Notice, you will be assigned with a confirmation number that will be used by your U.S. customs broker for the shipment release. A Prior Notice can also be filed by your own compnay or international transit firms as well as shipment companies (DHL, FedEx, TNT, etc).
U.S. FDA requires filling of Prior Notice before food shipments enter the United States. This will include Prior Notice for food samples for trade shows or consumption. After filling the Prior Notice, you will be assigned with a confirmation number that will be used by your U.S. customs broker for the shipment release. A Prior Notice can also be filed by your own compnay or international transit firms as well as shipment companies (DHL, FedEx, TNT, etc).
U.S. FDA requires filling of Prior Notice before food shipments enter the United States. This will include Prior Notice for food samples for trade shows or consumption. After filling the Prior Notice, you will be assigned with a confirmation number that will be used by your U.S. customs broker for the shipment release. A Prior Notice can also be filed by your own compnay or international transit firms as well as shipment companies (DHL, FedEx, TNT, etc).
Frequently Asked Questions (FAQ)
U.S. FDA requires filling of Prior Notice before food shipments enter the United States. This will include Prior Notice for food samples for trade shows or consumption. After filling the Prior Notice, you will be assigned with a confirmation number that will be used by your U.S. customs broker for the shipment release. A Prior Notice can also be filed by your own compnay or international transit firms as well as shipment companies (DHL, FedEx, TNT, etc).
U.S. FDA requires filling of Prior Notice before food shipments enter the United States. This will include Prior Notice for food samples for trade shows or consumption. After filling the Prior Notice, you will be assigned with a confirmation number that will be used by your U.S. customs broker for the shipment release. A Prior Notice can also be filed by your own compnay or international transit firms as well as shipment companies (DHL, FedEx, TNT, etc).
U.S. FDA requires filling of Prior Notice before food shipments enter the United States. This will include Prior Notice for food samples for trade shows or consumption. After filling the Prior Notice, you will be assigned with a confirmation number that will be used by your U.S. customs broker for the shipment release. A Prior Notice can also be filed by your own compnay or international transit firms as well as shipment companies (DHL, FedEx, TNT, etc).
Service Details & Fees
GUDID Account
395 USD
UDI Submission
195 USD
Time Required
4-6 Days
30-Min Expert Consultation
Discount on Multiple UDIs
_______ Related Services _______